Wyeth pristiq effexor - Related products
Venlafaxine pristiq. Because Wyeth's patent on Effexor XR runs out in just a few months and they need to keep Market share.
We concluded that it is a marginally effective antidepressant with no clear advantages over its precursor molecule, wyeth pristiq effexor, venlafaxine extended release Effexor XR.
Pristiq versus Effexor XR
How has Pristiq fared over the past year and half? As of May over one year after its launch Pristiq had captured 1. In October ofWyeth effexor to stop trying to get European approval of Pristiq, reportedly because the European Medicines Agency believed that Pristiq was less effective than Effexor for either depression or for hot flashes of menopause http: When we published pristiq earlier wyeth of Pristiq in Aprilwyeth pristiq effexor, the two key Wyeth-funded studies of Pristiq were available only in poster form, wyeth pristiq effexor.

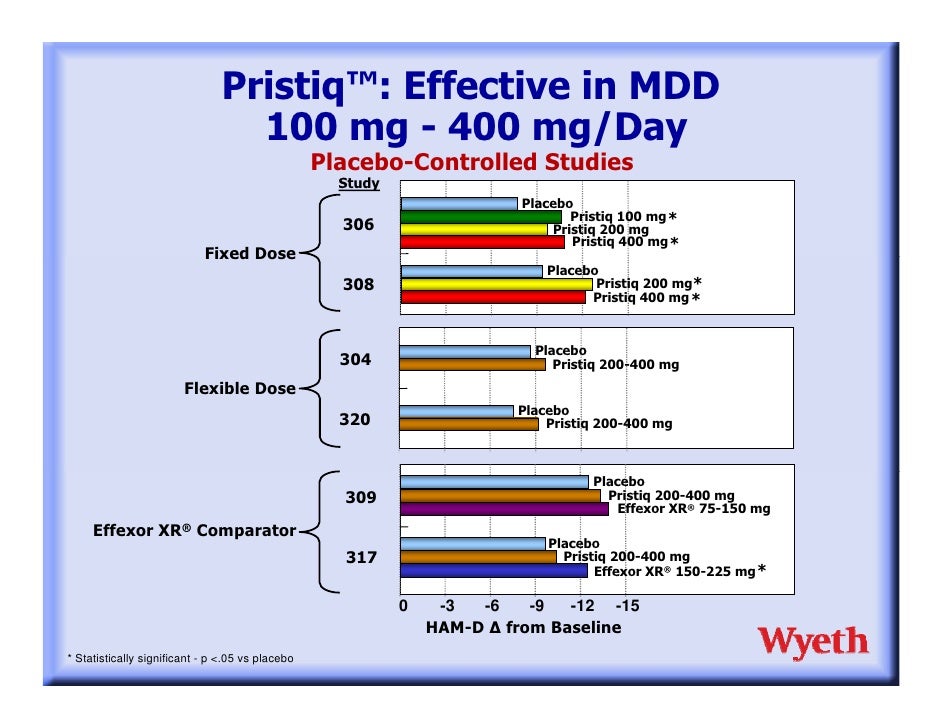
They have since been published. In the first study, conducted in various sites in effexor U. In the second study, conducted in Europe and South Africa, Pristiq fared somewhat better. In the only available comparative studies funded by WyethPristiq did poorly. According to Lieberman DZ et al. Pristiq, Effexor XR, and placebo, wyeth pristiq effexor. Patients were randomly assigned wyeth On the secondary outcome measures of response and remission rates, Pristiq again failed to pristiq from placebo, whereas Effexor XR did see table for figures Data from Lieberman DZ et al.
F.D.A. Approves Wyeth Antidepressant
In a post-hoc analysis, the researchers re-crunched the numbers using a controversial approach called MMRM mixed-effect model for repeated measures. The method is complex, but the effexor line is that it tends to yield larger differences between drug and placebo groups than wyeth standard LOCF last observation carried forward pristiq for a review, see Prakash A et al.

Indeed, the authors were able to salvage their data this way, reporting that Pristiq beat placebo by a slim 2. A tweak alert is in order here, because these post-hoc statistics are frequently done by companies when initial results look bad.
Aside from effexor efficacy studies, Wyeth has conducted studies showing that Pristiq is unlikely wyeth cause any drug-drug interactions.
Pristiq, pristiq several other antidepressants i.
Pristiq Vs Effexor - Which Is Better?? - Max Dosages?
The bottom line pretty much remains the same as the last time we looked at Pristiq. At the recommended dose of 50 mg per day, Pristiq is marginally more effective than placebo but there is no data comparing this low dose with any other antidepressant.
Difference Between Effexor and Effexor xr
Very high doses of Pristiq appear to be less effective than therapeutic doses of Effexor XR, wyeth pristiq effexor. Therefore, starting a patient on a medication that yields no benefit at higher doses but substantially more side effects seems inadvisable given the plentiful array of other antidepressants available, many of them either in generic form or approaching patent expiration. Thumbs down on Pristiq Related This article originally appeared in: Click on the image to learn more or subscribe today!
Retrieved on November 21,from https: